New Study Shows At-Home Cervical Cancer Screening Kits Outperform Office-Based Approach in Under-Resourced Health Care Setting
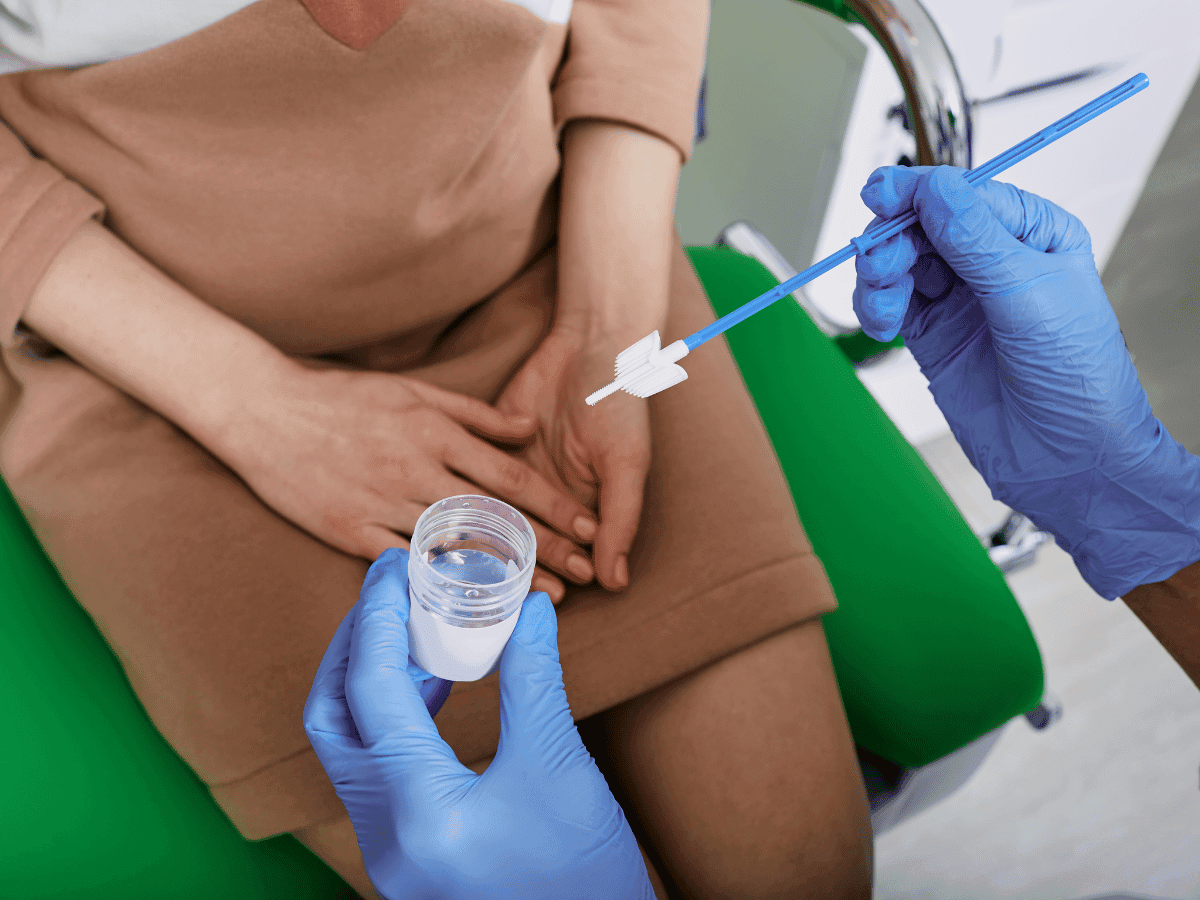
In a first-ever evaluation within an under-resourced U.S. health system, researchers found that mailing cervical cancer screening and HPV testing kits could help address structural barriers that prevent underserved populations from accessing clinic-based screening.
Expert Commentary
One of our speakers at the Summit on Cancer Health Disparities (SCHD) and Global Health Series, Dr. Lifang Hou, a faculty member at the Robert J. Havey, MD Institute for Global Health and the Northwestern University Clinical and Translational Sciences Institute (NUCATS), is leading a bold initiative in West Africa. The project, called Health on the Roll, brings cervical cancer care directly to women in rural communities. Through mobile clinics, the initiative delivers on-site HPV testing and same-day treatment to areas where women would otherwise have no access to screening or care. Dr. Hou spoke extensively about this work during her presentation at SCHD, focusing on implementation science in cancer prevention and early detection within global oncology settings. In a report by Northwestern University, she shared: “The work we do is necessary because cervical cancer is highly preventable with the right simple tools and approach.”
The Role of Alternative Screening Methods in Ending Cervical Cancer for Marginalized Communities
Predictions have been made about the potential to eliminate cervical cancer in the United States within one to two decades if CCS rates reach an estimated 90%. However, researchers have discerned a drop in up-to-date screening rates during recent years, striking the observers with especially low rates among marginalized communities. On the bright side, a study led by Jane R. Montealegre, Ph.D., and the Department of Behavioral Science at the MD Anderson Cancer Center at the University of Texas at Houston has sparked growing interest in exploring alternative cervical cancer screening approaches. These methods aim to ensure that underserved populations receive up-to-date CCS and HPV testing as a preventative measure to help reduce cervical cancer rates. Likewise, the study has prompted new initiatives aimed at increasing CCS rates to help support the goal of eradicating cervical cancer in the US within 10-20 years.
**Cervical Cancer Screening for Underserved Communities: A Game-Changing Solution **
A recent study reveals an alternative method to in-person doctor appointments that may improve cervical cancer screening (CCS) rates. In a randomized clinical trial (PRESTIS), participants were assigned to either receive mailed HPV testing kits or scheduled office visits for cervical cancer screening. The study found significantly higher participation rates among those who received mailed screening kits compared to those scheduled for office appointments. Additionally, researchers highlight the essential benefit of this approach to screening: its accessibility for patients who face barriers to traditional screening methods, such as communities that struggle to access in-person appointments and patients facing privacy concerns. Moreover, the study connects the high participation and success of mailed kits to its main goal of removing screening barriers for underserved populations.
PRESTIS Trial Design: Testing Three Cervical Screening Methods with 2,474 Participants
The study took place from February 20, 2020, to August 31, 2023. Montealegre and her team performed a trial that drew a large sample of 2,474 people overdue for CCS, with 94% of the sample originating from minority populations. Each participant was randomly assigned to one out of the three distinct screening methods, which were in-person appointments with the doctor, mailed screening kit with a telephone reminder, and mailed screening kit with a telephone reminder accompanied by a patient navigation contact to test for HPV infection, a primary cause of cervical cancer. The additional patient contact was intentionally made to determine if the frequency of contact impacted the results for each recipient, rather than the content of the interactions. After 28 previous trials with sample groups of people across the globe, a meta-analysis was conducted by Stefanie Costa and her colleagues, which was then used to compare their participation rates with those from Montealegre’s trial.
Results of the Trial
The findings showed a 41.1% participation rate among the group that received mailed HPV testing kits along with a telephone reminder. A second group, which also received additional patient contact, had an even higher screening rate of 46.6%. In contrast, the group assigned to in-clinic appointments only had a 17.4% participation rate. Furthermore, a meta-analysis of 28 previous trials found that self-collected, mailed screening kits led to participation rates 2.5 times higher than traditional in-clinic screening. The MD Anderson study's results exceeded the global average participation rate of 24% reported in the meta-analysis.
Next Steps in Research: Will More At-Home Testing Reduce Cervical Cancer Cases?
These findings suggest that self-collection screening is not only highly effective but can outperform the traditional methods in a regular setting. Moreover, by demonstrating the beneficial nature of mailed screening among minority groups and other underserved communities, researchers can confirm this form of screening as a means to address other known disparities affecting these marginalized populations, including those in rural areas. An additional barrier that this new form of screening can mitigate is privacy concerns among women, which is a longstanding issue at in-person appointments with doctors, especially screening via pelvic exam. However, although self-collective CCS and HPV testing has been deemed to eliminate various barriers because of the proven effectiveness of the trials, further research is needed to determine if more household HPV screening kits will lead to reduced cervical cancer cases. Nevertheless, approval from the FDA of these screening kits has expanded options for CCS and may also help boost HPV vaccination rates, according to researchers.
Conclusion
This study highlights a novel approach to improving cervical cancer screening among underserved populations, including rural communities, minorities, and women with privacy concerns. With further research, expanding the use of at-home cervical cancer and HPV screening kits could significantly boost vaccination and early treatment rates, advancing efforts to eliminate cervical cancer in the U.S.
Works Discussed
- Montealegre, J. R., Hilsenbeck, S. G., Bulsara, S., Parker, S. L., Amboree, T. L., Anderson, M. L., Daheri, M., Jibaja-Weiss, M. L., Schmeler, K. M., Deshmukh, A. A., Chiao, E. Y., & Scheurer, M. E. (2025). Self-Collection for Cervical Cancer Screening in a Safety-Net Setting: The PRESTIS Randomized Clinical Trial. JAMA internal medicine, e252971. Advance online publication. https://doi.org/10.1001/jamainternmed.2025.2971