More news
> All Top Headlines
Expert Opinion
See all »Written by Dr. Chepsy Philip from Believers Church Medical College Hospital, Tiruvalla, Kerala, India KEYNOTE-087 is a multicentre, single-arm, multicohort, nonrandomized, phase
MoreX-7/7 trial: Alternative fixed dosing of capecitabine associated with similar efficacy with better safety profile By Drs. Arya Mariam Roy &
MoreBy Dr. Aakash Desai Mayo Clinic KEYNOTE-789: No Significant Efficacy Benefit with Addition of Pembrolizumab to Chemotherapy in TKI-Resistant, EGFR-Mutated Metastatic
MoreBy Dr. Luke Fletcher Willamette Valley Cancer Institute & Research Center Low-risk MDS had many exciting updates, starting with the COMMANDS
MoreVideo News
See all »By Dr. Lei Deng University of Washington & Fred Hutch Watch Dr. Lei Deng’s overview of the KEYNOTE-671
MoreDr. Talha Badar of Mayo Clinic discusses the results of Quantum First, a randomized, phase III trial evaluating the efficacy of Quizartinib
MoreDr. Richa Parikh interviewed Dr. Shaji Kumar of Mayo Clinic about his recommendations for the treatment of myeloma.
MoreDr. Dipesh Uprety interviewed Dr. Chadi Nabhan about his new book, Toxic Exposure. Watch the video to hear more about the book
MoreNew Approvals
See all »By: Dr. Anish Shah Bronx-Lebanon Hospital; Bronx, NY On November 16, 2023, the U.S. Food and Drug Administration approved Pembrolizumab (Keytruda) plus fluoropyrimidine- and
MoreBy: Dr. Anish Shah Bronx-Lebanon Hospital; Bronx, NY On the date of approval, the U.S. Food and Drug Administration approved Nirogacestat (Ogsiveo) for adult
MoreBy: Dr. Anish Shah Bronx-Lebanon Hospital; Bronx, NY On November 16, 2023, the U.S. Food and Drug Administration approved capivasertib (Truqap, AstraZeneca Pharmaceuticals)
MoreBy: Dr. Anish Shah Bronx-Lebanon Hospital; Bronx, NY The U.S. Food and Drug Administration granted accelerated approval for Pirtobrutinib (Jaypirca) to be administered to
MorePatient Corner
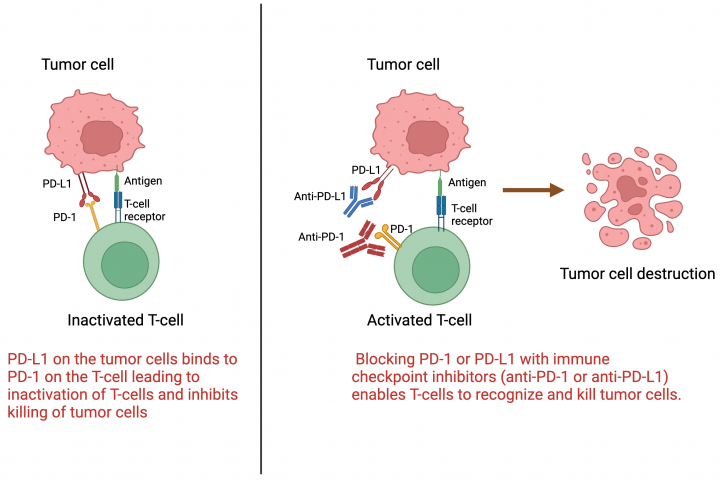
See all »By: Dr. Arya Mariam Roy Roswell Park Comprehensive Cancer Center What are immune checkpoint inhibitors? Immune checkpoint inhibitors are a type of
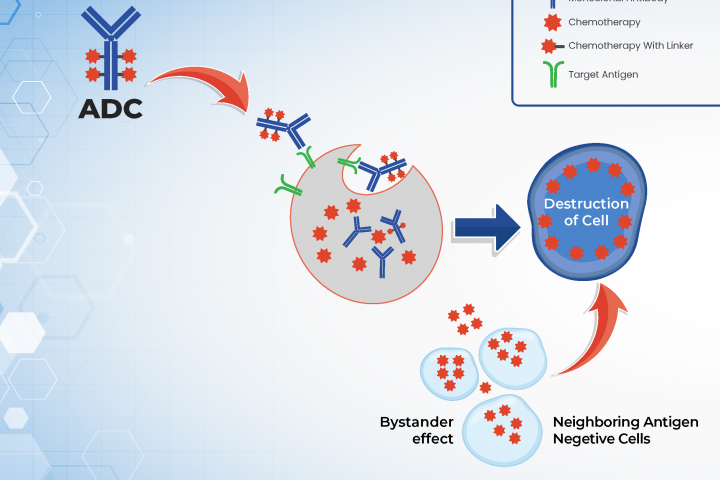
MoreAuthor: Dr. Arya Mariam Roy from Roswell Park Comprehensive Cancer Center What are antibody drug conjugates (ADCs)? Antibody drug conjugates (ADCs) are newer
More